- Slug: BC-CNS-FDA Try,820
- Photos available (thumbnails, captions below)
By JESSICA BOEHM
Cronkite News
WASHINGTON – The Food and Drug Administration announced plans this week to make it easier for Americans with terminal illnesses to access experimental drugs, shortening an application form from 100 hours to as little as 45 minutes.
The move was welcomed as a step in the right direction by advocates in Arizona who successfully pushed for a “Right to Try” law that grants state residents access to experimental drugs.
But only a very small step.
“The bottom line of this is that patients still have to ask the federal government for permission to save their own lives,” said Goldwater Institute President Darcy Olsen. “It just shortens the request forms.”
An FDA spokeswoman said in an email that the agency has approved 99 percent of the “compassionate use” requests it has received since 2010 using the old form.
“FDA believes the process works well overall, and has resources available to help physicians navigate the process,” said Sandy Walsh, the spokeswoman, in her email.
But the department is also “aware of concerns that some physicians who are unfamiliar with how to submit an Expanded Access request may be confused about what is required and therefore deterred from doing so,” she said. The proposed new application, which was posted Wednesday for a 60-day public comment period, aims to relieve those concerns.
Olsen is not impressed.
“This still leaves all of the control in the FDA’s hands and it still makes patients go through a middleman when they’re dying,” Olsen said.
The Goldwater Institute was a driving force behind last year’s ballot initiative to give people with life-threatening illnesses the “right to try.” The initiative was passed with more than 78 percent of the vote, meaning terminally ill patients in the state will not need special permission to try drugs that have completed phase 1 of a clinical trial but have not been approved for general use.
Arizona is one of five states with such a law, and Goldwater is working to pass “right to try” laws in other states.
Phoenix resident Paulina Morris also pushed for Arizona’s right to try law after her son Diego, then 11, woke up three years ago with a pain outside of his left knee.
“We literally though it was just another minor sports injury,” Morris said.
It wasn’t.
Diego was diagnosed with a rare bone disease. After surgery and chemotherapy, Morris was told by a physician about an experimental immunotherapy drug that could decrease the likelihood of the cancer spreading to other parts of Diego’s body. The drug had been approved for use in other countries, but not in the United States.
“We realized we had a choice,” Morris said. “Either stay here and not get this extra drug for Diego … or take him to another country.”
The family moved to Paris, where Diego spent nine months receiving 48 doses of the new drug.
Today, he is doing “fantastic,” his mother said – he was elected student body vice president and made the honor roll this year. But Diego and his family keep sharing their story so that other families will have a simpler process when trying to access experimental drugs.
“Worst-case scenario, I think, is to uproot your sick child and go to another country and take them away from everything familiar,” Morris said.
She said she was “really delighted that the FDA is moving a little bit on making it easier to access drugs.”
Another advocate agreed that the FDA’s move was a good one, but said the agency still has far to go.
Frank Burroughs is president of the Abigail Alliance for Better Access to Developmental Drugs, which has been pushing the FDA for years to shorten the application.
Burroughs said that when patients wanted to request access to developmental drugs before, they may have been unable to do so because “their doctors just did not have time to spend the 100 hours filling out the paperwork – they’d neglect their other patients.”
His own daughter, Abigail, was unable to get access to an experimental drug that was performing well in early tests to treat head and neck cancer. The drug was approved for general use to treat that type of cancer 4.5 years after Abigail’s 2001 death, Burroughs said.
A few hours after Abigail died, Burroughs decided he would keep working to expand early access to developmental drugs.
“Why should I quit now?” he asked. “There are other people as precious as Abigail.”
Burroughs said the FDA’s streamlined application is “a step in the right direction” and that it will help between 2,000 and 4,000 people.
But he has his sights set on bigger goals now: The Abigail Alliance will continue to push for earlier approval of drugs, he said.
“With modern science and technology that we have now, they can know earlier how well a drug is working, how it’s working even down to the cell molecular level,” Burroughs said.
^__=
Web links:
_ FDA blog post about the announcement: http://blogs.fda.gov/fdavoice/index.php/2015/02/a-big-step-to-help-the-patients-most-in-need/
_ Proposed new form: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM432717.pdf
_ Goldwater Institute Right to Try page: http://goldwaterinstitute.org/en/work/topics/healthcare/right-to-try/right-try/
_ FDA’s record of approvals: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/INDActivityReports/ucm373560.htm
_ Prop 303: http://www.azsos.gov/election/2014/Info/PubPamphlet/english/prop303.htm
_ 2014 Arizona election results: http://www.azsos.gov/election/2014/General/Canvass2014GE.pdf
_ Abigail Alliance: http://www.abigail-alliance.org/
^__=
Phoenix resident Paulina Morris, in a November photo, took her son, Diego, to Paris for medication to treat his cancer, because the drug was available in France but had not been approved in the U.S. (Cronkite News photo by Sierra Oshrin)
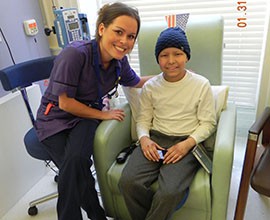
Diego, here with a nurse during treatment three years ago for a rare bone disease, has recuperated and it doing “fantastic,” his mother said, and is back in school and on the honor roll. (Photo courtesy Paulina Morris)
